Pediatric Post-Resuscitation Care Algorithm: Return of Spontaneous Circulation (ROSC)

Cardiac arrest in a pediatric patient is a critical event that requires prompt recognition and intervention to restore circulation. High-quality cardiopulmonary resuscitation (CPR) and defibrillation as needed are key first steps, but the management does not stop there. Once the return of spontaneous circulation (ROSC) is achieved, a structured approach is necessary to stabilize the patient and optimize recovery.
The post-resuscitation phase starts immediately after ROSC and addresses several key goals: maintaining oxygenation and ventilation, managing hemodynamics, and preventing secondary organ injury. The highest priority is to preserve neurologic function. Pediatric patients are at high risk for neurologic function. Pediatric patients are at high risk for neurologic impairment following cardiac arrest.
Post-resuscitation care requires a coordinated, multidisciplinary approach. The pediatric intensive care team works closely with cardiology, neurology, respiratory therapy, and other services to provide integrated care based on established algorithms. These algorithms aim to restore and maintain adequate end-organ perfusion, particularly to the brain. The pediatric post-resuscitation care algorithm follows the below process:
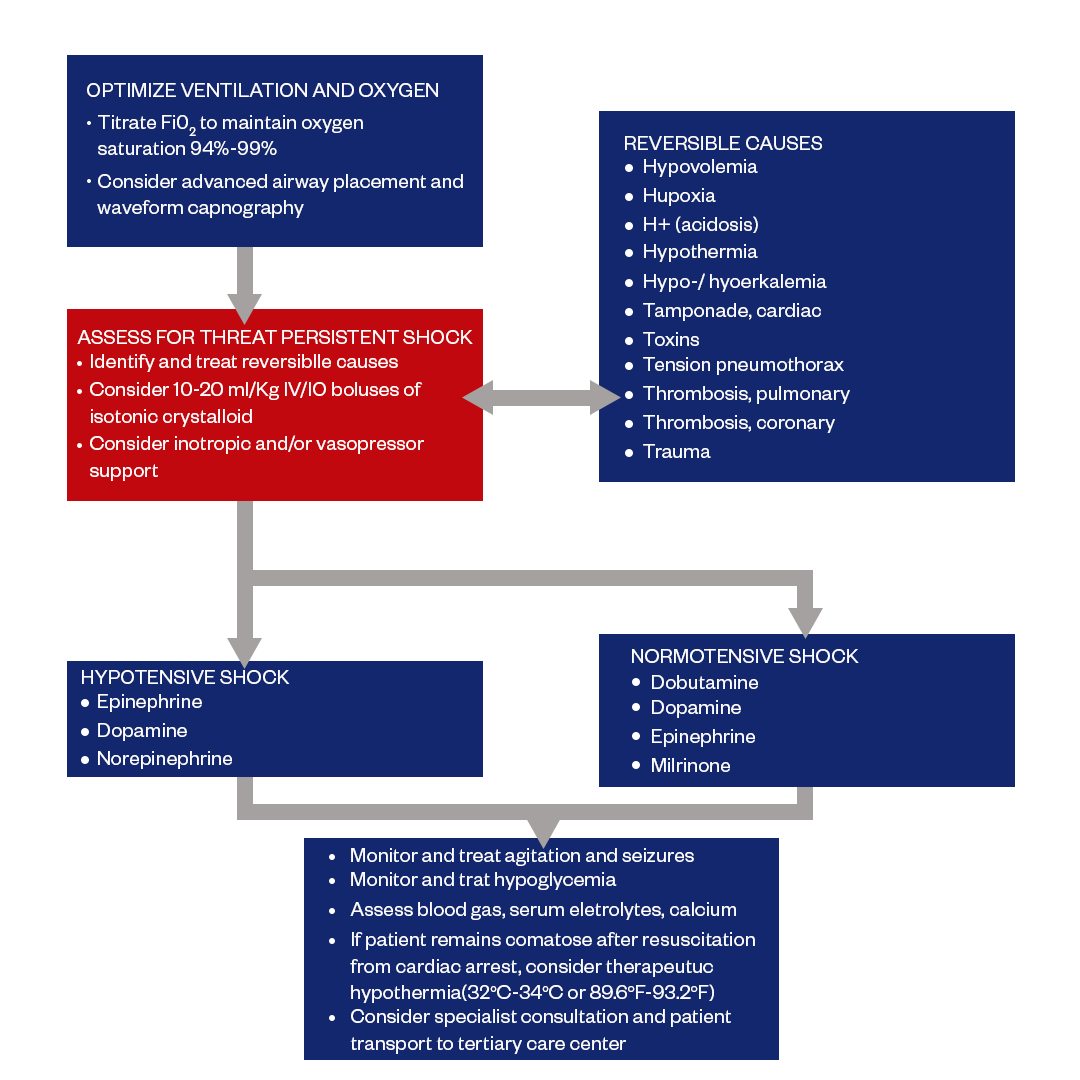
Figure: Pediatric Post Resuscitation Care Algorithm
Optimize Oxygenation and Ventilation
Oxygenation and ventilation are the first priorities after ROSC, as they directly impact delivery of oxygen to vital organs, particularly the brain. Even brief episodes of hypoxemia or hypercarbia can be detrimental.
Maintaining normal oxygenation and ventilation is a critical component of post-resuscitation care. Airway intervention should be prioritized immediately after ROSC. meticulous lung-protective ventilation aims to restore adequate gas exchange, optimize cerebral perfusion, and prevent further secondary injury.
The goals are to ensure adequate gas exchange and avoid hypoxemia, hyperoxia, and hypocapnia/hypercapnia. This requires definitive airway control with endotracheal intubation and mechanical ventilation.
How to assess?
- Endotracheal intubation should be performed soon after ROSC if the patient is unable to protect their airway. This allows precise control of FiO2 and ventilation parameters.
- Initial ventilator settings:
- FiO2 100% to ensure oxygenation, then titrate to SpO2 92-97% to avoid hyperoxia
- Normocapnia (PaCO2 35-45 mmHg) helps optimize cerebral blood flow
- Continuous pulse oximetry, end-tidal CO2 monitoring, and serial arterial blood gases help guide adjustments to FiO2 and respiratory rate/tidal volumes.
- Sedation and/or paralytics may be needed to ensure lung-protective ventilation and patient-ventilator synchrony.
- Respiratory support will be required for 24 hours or longer following cardiac arrest to allow recovery. WeanFiO2 and ventilation parameters gradually.
Appropriate ETT Placement
ETT refers to “Endotracheal Tube.” It’s a medical device that is used to provide a pathway for adequate air to reach the lungs, the device is placed between vocal cords through the trachea to protect the contamination.
After endotracheal intubation, proper tube position must be ensured. Incorrect placement can lead to unilateral lung ventilation, aspiration, or accidental extubation.
How to assess?
- Confirm appropriate tube depth based on patient size and age (shown in the table below). The tip should be placed above the carina, halfway between the clavicles.
|
|
Age Group | Distance from tube tip to carina in the shortest trachea of age group (mm) |
|---|---|---|
| 3 | Newborns to <1 yr | 15.4 |
| 3.5 | 1 to <2 yr | 16.0 |
| 4 | 2 to <4 yr | 16.6 |
| 4.5 | 4 to <6 yr | 19.8 |
| 5 | 6 to <8 yr | 22.0 |
| 5.5 | 8 to <10 yr | 23.2 |
| 6 | 10 to <12 yr | 25.4 |
| 6.5 | 12 to <14 yr | 28.5 |
| 7 | 14 to <16 yr | 31.7 |
- Check for bilateral breath sounds to confirm ventilation to both lungs. Listen over the epigastrium to ensure there are no gastric sounds.
- Secure the ETT well to prevent displacement. Immobilize the head/neck.
- Obtain a chest x-ray to verify the correct placement above the carina. Look for symmetrical aeration of lung fields.
- Monitor end-tidal CO2 waveform continuously. Abrupt loss may indicate tube dislodgement.
- Reassess tube position frequently, with every patient move, and after tracheal suctioning.
Ensuring appropriate tube depth and position is a key step after intubation. Proper placement in the mid-trachea allows optimal oxygenation and ventilation to both lungs. It also prevents inadvertent extubation or right mainstem intubation. Regular reassessments help detect any ETT dislodgement early. Correct tube position is vital for adequate gas exchange during post-resuscitation care.
Optimize Cardiac Output
Restoring adequate organ perfusion is another vital goal after ROSC. Cardiac arrest causes global ischemia and reperfusion injury. The heart’s pumping function may be impaired after resuscitation.
Optimizing cardiac output is key to maintaining perfusion pressure and preventing secondary ischemic injuries. Aggressive hemodynamic monitoring guides the use of fluids, vasopressors/inotropes, and cardiovascular support needed to stabilize the post-arrest patient.
How to assess?
- Optimizing cardiac output helps maintain end-organ perfusion and prevents hypotension. The goal is to maintain systolic blood pressure >5th percentile for age.
- Invasive hemodynamic monitoring (arterial line, central venous pressure) is ideal if available.
- Give 10-20 mL/kg isotonic crystalloid fluid boluses for suspected hypovolemia or hypotension. Avoid fluid overload.
- Continuous cardiac rhythm monitoring and echocardiography help evaluate myocardial function and guide therapy.
- Vasopressors like dopamine and epinephrine can help increase systematic vascular resistance and blood pressure if fluids are inadequate. Start at 5-10mcg/kg/min and titrate to effect.
- Inotropes like dobutamine and milrinone may be needed for myocardial dysfunction with low cardiac output.
- Vasopressin can be considered for vasodilation unresponsive to catecholamines.
Increase Preload by Administering Fluid Boluses
Pediatric patients are at high risk of developing hypovolemic shock after cardiac arrest due to global ischemia/reperfusion injury. Fluid resuscitation helps increase preload and optimize cardiac output.
How to assess?
- Suspect hypovolemia in any post-arrest patient with hypotension, poor perfusion, or high systemic vascular resistance.
- Give 10-20 mL/kg isotonic crystalloid fluid boluses (normal saline or lactated Ringer's solution) over 10-20 minutes. Reassess after each bolus.
- The goal is to improve blood pressure, urine output, and other markers of end-organ perfusion. Avoid fluid overload.
- Monitor for signs of fluid overload - increased work of breathing, rales, gallop rhythm, hepatomegaly. Diuretics may be needed if fluid overload develops.
- If shock persists despite 40-60 mL/kg fluid, add vasopressor therapy for additional hemodynamic support.
Fluid resuscitation is a first-line intervention for post-arrest shock before adding vasoactive medications. Timely fluid boluses help restore intravascular volume, increase stroke volume and cardiac output, and improve tissue perfusion. Ongoing reassessment determines the need for further fluid/vasopressor therapy to optimize perfusion.
Optimize Neurologic Outcome
The ultimate goal of post-resuscitation care is optimizing neurologic outcomes by preventing secondary brain injury. Children are at high risk of hypoxic-ischemic encephalopathy (HIE) after cardiac arrest.
Preventing secondary neurologic injury is key for intact survival. Strict avoidance of hypotension, along with optimal oxygenation, ventilation, and cerebral perfusion, is critical to protecting the post-ischemic brain. Hypothermia and neurocritical care input may further improve outcomes.
How to assess?
- Maintain euvolemia and adequate perfusion pressure to prevent hypotension and cerebral hypoperfusion.
- Target normal PaO2 and PaCO2 levels to optimize cerebral blood flow. Avoid hypoxemia, hyperoxia, hypocapnia,, or hypercapnia.
- Consider therapeutic hypothermia (32-34°C for 24 hours) in comatose patients to reduce neurologic injury. Rewarm slowly.
- Control seizures with anticonvulsants; treat promptly to prevent further neuronal damage.
- Frequently monitor neurologic status with examinations. Check for pupil reactivity.
- Obtain urgent neuroimaging (CT or MRI head) if any deterioration to evaluate for cerebral edema, hematomas, or stroke.
- Consult neurology early. They can provide prognosis and guide additional neuroprotective strategies and supportive care.
- Discuss goals of care with family if prognosis is poor; consider limits on life-sustaining treatment.
Fast and Convenient
Take PALS Classes
*Nationally Accepted
PALS
CERTIFICATION
AHA PALS course
State-of-the-Art Facilities
Unlimited Exam Retakes
$260
PALS
ONLINE CERTIFICATION
AHA PALS Online Course
100% online training
Unlimited Exam Retakes
$300